what is the reaction that corresponds to the first ionization energy of rubidium, rb
Ionization Free energy and Electron Analogousness
The First Ionization Free energy
The energy needed to remove one or more than electrons from a neutral atom to class a positively charged ion is a concrete property that influences the chemic behavior of the atom. Past definition, the first ionization energy of an element is the energy needed to remove the outermost, or highest free energy, electron from a neutral atom in the gas phase.
The procedure by which the first ionization energy of hydrogen is measured would be represented by the following equation.
The magnitude of the first ionization free energy of hydrogen tin can be brought into perspective by comparing it with the energy given off in a chemical reaction. When we burn natural gas, well-nigh 800 kJ of energy is released per mole of methyl hydride consumed.
The thermite reaction, which is used to weld iron track, gives off nigh 850 kJ of energy per mole of iron oxide consumed.
The start ionization energy of hydrogen is half once again as large as the energy given off in either of these reactions.
![]()
Patterns in the First Ionization Energies
The get-go ionization energy for helium is slightly less than twice the ionization energy for hydrogen because each electron in helium feels the attractive force of two protons, instead of i.
It takes far less energy, however, to remove an electron from a lithium atom, which has iii protons in its nucleus.
This can be explained by noting that the outermost, or highest energy, electron on a lithium cantlet is in the twos orbital. Considering the electron in a 2s orbital is already at a higher free energy than the electrons in a 1due south orbital, it takes less free energy to remove this electron from the atom.
The kickoff ionization energies for the main group elements are given in the two figures below.
2 trends are apparent from these information.
- In general, the offset ionization energy increases equally nosotros go from left to right beyond a row of the periodic table.
- The get-go ionization free energy decreases as we go down a column of the periodic table.
The first trend isn't surprising. We might look the commencement ionization energy to become larger as we get beyond a row of the periodic table because the force of attraction betwixt the nucleus and an electron becomes larger as the number of protons in the nucleus of the atom becomes larger.
The 2d tendency results from the fact that the primary quantum number of the orbital holding the outermost electron becomes larger as nosotros become down a column of the periodic table. Although the number of protons in the nucleus also becomes larger, the electrons in smaller shells and subshells tend to screen the outermost electron from some of the forcefulness of attraction of the nucleus. Furthermore, the electron being removed when the first ionization energy is measured spends less of its time near the nucleus of the atom, and information technology therefore takes less energy to remove this electron from the cantlet.
![]()
Exceptions to the General Pattern of Beginning Ionization Energies
The figure below shows the first ionization energies for elements in the second row of the periodic tabular array. Although there is a full general tendency toward an increment in the first ionization energy as we go from left to right beyond this row, there are two modest inversions in this pattern. The beginning ionization free energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen.
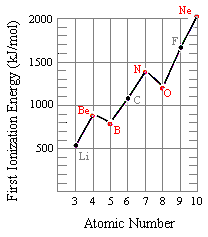
These observations tin can be explained past looking at the electron configurations of these elements. The electron removed when a beryllium atom is ionized comes from the iisouth orbital, but a iip electron is removed when boron is ionized.
Be: [He] twosouth 2
B: [He] 2s 2 twop 1
The electrons removed when nitrogen and oxygen are ionized besides come from 2p orbitals.
N: [He] 2s 2 twop iii
O: [He] 2southward 2 iip 4
Simply in that location is an of import departure in the way electrons are distributed in these atoms. Hund'southward rules predict that the three electrons in the 2p orbitals of a nitrogen atom all have the same spin, but electrons are paired in one of the 2p orbitals on an oxygen atom.

Hund's rules can exist understood by bold that electrons try to stay as far autonomously every bit possible to minimize the force of repulsion betwixt these particles. The three electrons in the iip orbitals on nitrogen therefore enter different orbitals with their spins aligned in the same direction. In oxygen, two electrons must occupy 1 of the 2p orbitals. The force of repulsion between these electrons is minimized to some extent by pairing the electrons. At that place is still some rest repulsion betwixt these electrons, however, which makes information technology slightly easier to remove an electron from a neutral oxygen cantlet than we would expect from the number of protons in the nucleus of the atom.
![]()
2d, Third, Fourth, and Higher Ionization Energies
Past now yous know that sodium forms Na+ ions, magnesium forms Mg2+ ions, and aluminum forms Althree+ ions. But have you lot e'er wondered why sodium doesn't form Naii+ ions, or even Na3+ ions? The answer can be obtained from data for the 2d, third, and higher ionization energies of the element.
The first ionization energy of sodium, for example, is the energy information technology takes to remove one electron from a neutral atom.
Na(thousand) + free energy ![]() Na+(g) + e-
Na+(g) + e-
The second ionization energy is the free energy it takes to remove another electron to form an Natwo+ ion in the gas phase.
Na+(chiliad) + energy ![]() Na2+(g) + east-
Na2+(g) + east-
The tertiary ionization energy can be represented by the following equation.
Na2+(k) + energy ![]() Na3+(g) + e-
Na3+(g) + e-
The energy required to form a Na3+ ion in the gas phase is the sum of the first, 2d, and third ionization energies of the chemical element.
Get-go, Second, Third, and Fourth Ionization Energies
of Sodium, Magnesium, and Aluminum (kJ/mol)

Information technology doesn't take much free energy to remove one electron from a sodium atom to form an Na+ ion with a filled-shell electron configuration. One time this is washed, withal, it takes almost 10 times every bit much energy to interruption into this filled-shell configuration to remove a second electron. Because information technology takes more than energy to remove the second electron than is given off in any chemical reaction, sodium can react with other elements to form compounds that contain Na+ ions but not Natwo+ or Na3+ ions.
A similar design is observed when the ionization energies of magnesium are analyzed. The first ionization free energy of magnesium is larger than sodium considering magnesium has one more than proton in its nucleus to hold on to the electrons in the 3south orbital.
Mg: [Ne] 3s 2
The 2nd ionization energy of Mg is larger than the first because it always takes more than energy to remove an electron from a positively charged ion than from a neutral atom. The third ionization energy of magnesium is enormous, nevertheless, considering the Mg2+ ion has a filled-beat out electron configuration.
The same design can be seen in the ionization energies of aluminum. The first ionization energy of aluminum is smaller than magnesium. The 2d ionization energy of aluminum is larger than the commencement, and the tertiary ionization free energy is even larger. Although information technology takes a considerable amount of energy to remove three electrons from an aluminum atom to form an Al3+ ion, the energy needed to pause into the filled-beat configuration of the Al3+ ion is astronomical. Thus, it would be a error to wait for an Aliv+ ion as the production of a chemical reaction.
Predict the group in the periodic table in which an chemical element with the following ionization energies would most likely be plant.
1st IE = 786 kJ/mol
2nd IE = 1577
3rd IE = 3232
4th IE = 4355
5th IE = 16,091
sixth IE = nineteen,784
Click hither to check your answer to Practice Problem 5
Use the trends in the ionization energies of the elements to explain the following observations.
(a) Elements on the left side of the periodic table are more likely than those on the right to form positive ions.
(b) The maximum positive charge on an ion is equal to the group number of the element
Click hither to check your respond to Practice Problem vi
![]()
Electron Affinity
Ionization energies measure the tendency of a neutral cantlet to resist the loss of electrons. It takes a considerable amount of energy, for example, to remove an electron from a neutral fluorine atom to form a positively charged ion.
The electron affinity of an element is the energy given off when a neutral cantlet in the gas phase gains an actress electron to class a negatively charged ion. A fluorine atom in the gas phase, for example, gives off free energy when information technology gains an electron to grade a fluoride ion.
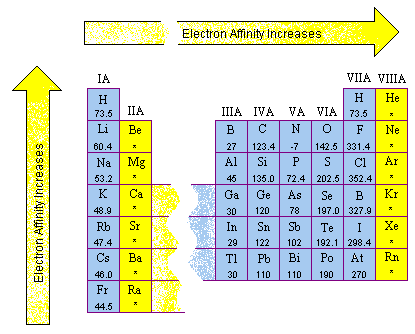
Electron affinities are more than hard to measure out than ionization energies and are ordinarily known to fewer significant figures. The electron affinities of the main group elements are shown in the figure below.
Several patterns can be found in these data.
- Electron affinities by and large become smaller as nosotros become downwards a column of the periodic table for two reasons. Offset, the electron being added to the cantlet is placed in larger orbitals, where it spends less time almost the nucleus of the atom. Second, the number of electrons on an atom increases as we go down a column, so the force of repulsion between the electron beingness added and the electrons already present on a neutral cantlet becomes larger.
- Electron affinity data are complicated by the fact that the repulsion between the electron being added to the atom and the electrons already nowadays on the atom depends on the volume of the atom. Among the nonmetals in Groups VIA and VIIA, this strength of repulsion is largest for the very smallest atoms in these columns: oxygen and fluorine. Equally a result, these elements accept a smaller electron affinity than the elements below them in these columns as shown in the figure below. From that point on, however, the electron affinities decrease as we continue down these columns.

At commencement glance, in that location appears to be no pattern in electron affinity across a row of the periodic tabular array, as shown in the figure below.
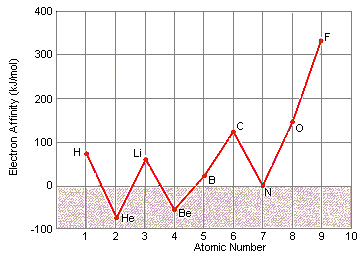
When these data are listed along with the electron configurations of these elements, withal, they make sense. These data can be explained by noting that electron affinities are much smaller than ionization energies. As a upshot, elements such as helium, glucinium, nitrogen, and neon, which have unusually stable electron configurations, have such small-scale affinities for extra electrons that no free energy is given off when a neutral atom of these elements picks up an electron. These configurations are and then stable that information technology actually takes energy to forcefulness one of these elements to pick up an extra electron to class a negative ion.
Electron Affinities and Electron Configurations for the Get-go 10 Elements in the Periodic Table
| Chemical element | Electron Affinity (kJ/mol) | Electron Configuration | |||||
| H | 72.8 | 1s i | |||||
| He | <0 | 1s two | |||||
| Li | 59.8 | [He] twos 1 | |||||
| Be | <0 | [He] twosouth two | |||||
| B | 27 | [He] 2due south two 2p i | |||||
| C | 122.iii | [He] twodue south 2 iip two | |||||
| N | <0 | [He] 2s 2 2p 3 | |||||
| O | 141.1 | [He] 2s 2 2p iv | |||||
| F | 328.0 | [He] 2s two 2p 5 | |||||
| Ne | <0 | [He] 2due south ii 2p 6 | |||||
![]()
Consequences of the Relative Size of Ionization Energies and Electron Affinities
Students often believe that sodium reacts with chlorine to form Na+ and Cl- ions because chlorine atoms "like" electrons more than sodium atoms do. In that location is no doubtfulness that sodium reacts vigorously with chlorine to form NaCl.
2 Na(s) + Clii(g) ![]() two NaCl(s)
two NaCl(s)
Furthermore, the ease with which solutions of NaCl in water conduct electricity is evidence for the fact that the production of this reaction is a salt, which contains Na+ and Cl- ions.
| NaCl(southward) | H2O | Na+(aq) + Cl-(aq) |
The only question is whether it is legitimate to presume that this reaction occurs because chlorine atoms "similar" electrons more than than sodium atoms.
The offset ionization energy for sodium is one and one-half times larger than the electron affinity for chlorine.
Na: 1st IE = 495.8 kJ/mol
Cl: EA = 328.8 kJ/mol
Thus, information technology takes more free energy to remove an electron from a neutral sodium atom than is given off when the electron is picked up by a neutral chlorine atom. We will obviously take to notice some other explanation for why sodium reacts with chlorine to form NaCl. Before nosotros can exercise this, yet, we demand to know more about the chemistry of ionic compounds.
![]()
Source: https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch7/ie_ea.php
0 Response to "what is the reaction that corresponds to the first ionization energy of rubidium, rb"
Post a Comment